 Currently, the only approved drugs for Alzheimer’s merely alleviate some of the symptoms — partially and temporarily — but do not stop the disease from progressing. (Shutterstock)
Currently, the only approved drugs for Alzheimer’s merely alleviate some of the symptoms — partially and temporarily — but do not stop the disease from progressing. (Shutterstock)
As a researcher who studies Alzheimer’s disease and a neurologist who cares for people with Alzheimer’s, I share in the frustration, indeed anger, of people and families when I tell them that I have no cure to offer.
Over the past year, scientists have tackled COVID-19, a previously unknown disease and within months developed effective new vaccines. Over that same time frame, the list of Alzheimer’s treatment failures got longer. Currently, the only approved drugs for Alzheimer’s merely alleviate some of the symptoms — partially and temporarily — but do not stop the disease from progressing.
Although it was first officially described 115 years ago, and of course existed long before that, we still do not have a cure for this devastating disease. Why?
Let’s start by following the money. For years, patient advocates have pointed to the escalating toll and ballooning costs of Alzheimer’s as the world’s population ages. Alzheimer’s is severely underfunded in comparison to cancer, heart disease, HIV/AIDS and even COVID-19.
Get The Latest By Email
Sadly, the mistaken belief that Alzheimer’s only affects older people is a contributing factor to this underfunding. However, five to 10 per cent of people with Alzheimer’s are under 65 years of age; some are even in their 40s. Alzheimer’s is also a disease of the entire family, causing anxiety, depression and exhaustion in caregivers and loved ones, exacting a disproportionately high socio-economic cost.
Conflicting theories
Funding is not the only issue here. The human brain is extremely complex, and Alzheimer’s disease is the most complex disease of the brain. The challenges that arise from this collision of complexities are reflected by the many competing theories of Alzheimer’s.
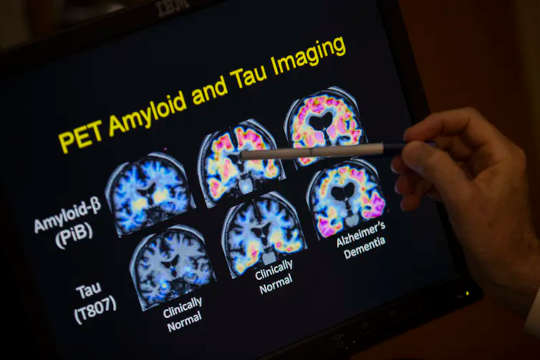
The most time-honoured theory is that Alzheimer’s is caused by misfolded proteins that aggregate or clump, killing brain cells and giving rise to the symptoms of memory loss and reduced cognition. Initially, the culprit in this misfolding story was a protein called beta-amyloid. More recently, another protein, tau, has emerged as a possible contributor.
 The protein misfolding behind Alzheimer’s disease may involve beta-amyloid or tau proteins. (AP Photo/Evan Vucci)
The protein misfolding behind Alzheimer’s disease may involve beta-amyloid or tau proteins. (AP Photo/Evan Vucci)
Although a wealth of research data have supported this protein misfolding theory, referred to as the amyloid hypothesis, multiple drugs designed to block the brain’s toxic protein misfolding processes have failed in human trials, repeatedly. In fact, in the past two years, several major clinical trials based on the field’s leading hypothesis — that reducing the level of aggregated beta-amyloid that riddles the brains of Alzheimer’s patients would halt disease progression — have dramatically failed.
And so there are many other theories. A new heavyweight contender is the neuroinflammation theory of Alzheimer’s which suggests that the disease arises from an excessive release of toxic inflammatory chemicals from immune cells in the brain called microglia. Drugs designed to address this theory are fundamentally different from those addressing the amyloid hypothesis, and are still early in the developmental process.
A different theory claims that Alzheimer’s is a disease of synapses, which are the junctions between brain cells, and yet another suggests that Alzheimer’s is a disease of mitochondria, a structure central to energy production in every brain cell.
Challenges to finding a cure
The path towards a cure is not going to be easy, and even if these theories do lead to the development of drugs, these drugs may fail for a host of other reasons.
Alzheimer’s is a very long, chronic disease, probably present 20 to 30 years before the first symptoms become obvious. Giving the drug when a person becomes symptomatic may be too late for it to make any difference. But we do not have the ability to diagnose it 30 years before the first symptoms, and even if we could, we would need to consider the ethics of giving a potentially toxic drug long-term to someone who may or may not get a disease in three decades.
Also, unlike developing antibiotics in which the researchers know within days if the drug works, the chronic nature of Alzheimer’s requires long, expensive trials — years in duration — before an answer can be attained. Such time and expense is a further impediment to drug development.
One final problem is that Alzheimer’s may not simply be one disease. It may in fact be a collection of similar diseases. A 52-year-old with early onset Alzheimer’s certainly has a clinical course distinct and different from an 82-year-old with late onset Alzheimer’s. Will a drug that works in an 82-year-old also work in a 52-year-old person’s disease? Maybe, or maybe not.
Thankfully, despite these many hurdles, a wealth of fascinating and encouraging research is taking place in laboratories around the world. The successes of science and the pharmaceutical industry against many other diseases over the past century have often arisen from picking low-hanging fruit. Alzheimer’s disease is not a low-hanging fruit, but the apple at the very top of the tree, and scientists are going to have to climb a lot of branches — many of which have never been trodden upon — on the way to a cure. But we’ll get there.![]()
About The Author
Donald Weaver, Professor of Chemistry and Director of Krembil Research Institute, University Health Network, University of
books_health
This article is republished from The Conversation under a Creative Commons license. Read the original article.







