It is estimated that there is a staggering £300m worth of medicine unused in the UK every year. But is it safe to take these medicines if they are past their expiry date?
Expiry dates are put in place after rigorous trialling and controlled experiments to ensure the safety and effectiveness of the drugs people take. In short, they guarantee the potency of the drug. Medicinal drugs are all chemicals and the rate at which they go off will depend upon their chemical structure, the drug preparation, how they are packaged, environmental conditions, whether they are subject to microbial contamination and their exposure to heat, light, oxygen and water.
The drugs are sold in a variety of containers including bottles, blister packs, tubes and ampules. They are relatively secure while sealed. But once the seal is broken, the process of “going off” accelerates.
Common painkillers
Let’s look at an everyday drug like paracetamol. This is an “over the counter” medicine, freely available, which helps to reduce pain or a fever. Paracetamol is sometimes sold in brown sealed bottles. The seal keeps moisture and atmospheric oxygen out. The brown bottle keeps UV light out as this can also cause the drug to breakdown. Once the seal is broken, the tablets are exposed to water and oxygen in the air and breakdown begins.
Get The Latest By Email
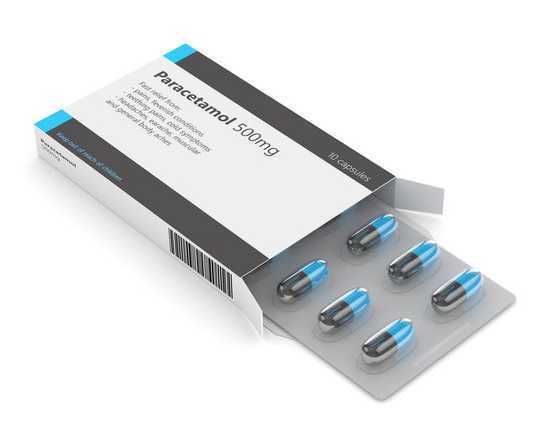 Paracetamol comes in bottles and blister packs and can be bought over the counter. Shutterstock/AleksandraGigowska
Paracetamol comes in bottles and blister packs and can be bought over the counter. Shutterstock/AleksandraGigowska
Paracetamol is also sold in blister packs. These packs are permeable to water and oxygen so they are covered in, for example, polyvinylidene chloride (PVDC). This protects the contents and slows down the decomposition process but is still slightly permeable so doesn’t prevent it altogether.
The consequence of this is that, despite the protection of the packaging, the drug content slowly declines. Research shows that when paracetomol based medicines go past their expiry date, up to 30% of the drug may break down in between 12 and 24 months.
Cold remedies and antibiotics
Cold remedies contain both paracetamol and a decongestant (usually phenylephrine hydrochloride). These can be sold as powders, capsules, oral solutions or nasal sprays.
While drugs in dry powder and capsule form may be relatively stable, those in liquid form may go off more quickly. For example, cold remedies such as nasal sprays contain both preservatives and antioxidants that only work at lower temperatures. They are considerably less effective above 40℃.
Similarly, antibiotics such as amoxicillin and erythromycin can be prescribed as an oral suspension in water. The dry powder form of the drug is, again, relatively stable. But the shelf life of the drug mixed with water by the pharmacist may only be seven to ten days – even when kept in a fridge.
Some drugs in liquid form have a considerably shorter shelf life. For example, nitroglycerin (glyceryltrinitrate) is used to treat angina and coronary heart disease. Formulations include liquids, tablets and capsules. Nitrate esters, of which this drug is an example, readily break down in the presence of water, rendering the drug ineffective.
Some formulations of nitroglycerin contain stabilisers to reduce this and are contained in protective packaging. But even these formulations only have a shelf life of about three months. Users often divide up their doses into pill boxes. The shelf life of nitroglycerin, once out of protective packaging, is reduced down to less than a week because of the rapid rate of drug breakdown.
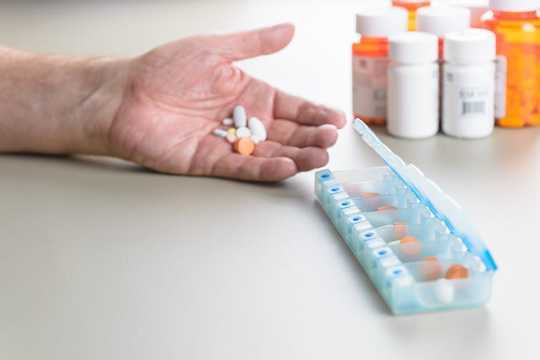 Taking pills out of their packs and putting them in boxes can reduce their shelf life. Shutterstock/JeffBaumgart
Taking pills out of their packs and putting them in boxes can reduce their shelf life. Shutterstock/JeffBaumgart
Even large, macromolecular drugs, like insulin, present problems. Insulin is a polypeptide used in the maintenance of blood glucose levels and the management of diabetes.
The drug is dissolved in water and when stored in a refrigerator (typically around 4℃) spoiling can be slowed. The solutions also contain preservatives to reduce the rate of spoiling. However, as they are small proteins, the drug molecules may break down in water, and in some instances bacteria can start to grow and break down the protein. This is why the shelf life of drugs such as insulin is very limited.
Safety
So is it safe to take medicines after their expiration date? The answer depends on the drug in question but, generally, no. Drugs like nitroglycerin may, in some instances, be life saving. But the actual drug content of out-of-date medicines such as this may mean that there is no effective medicine left in what is being taken. So there will be no effect on the target condition.
In the case of liquid antibiotics the concentration of the drug may be so reduced that it is not effective. To make things worse, studies have shown that the bacteria the antibiotic is being used to treat may, at lower concentrations of the drug, develop resistance that could render the antibiotic ineffective.
In other cases, like paracetamol, the consequences may not be so severe. But the drug content won’t be known. So if in doubt, check with pharmacists and doctors and try your best to keep drugs in date.![]()
About The Author
Michael Cole, Professor of Forensic Science, Anglia Ruskin University
This article is republished from The Conversation under a Creative Commons license. Read the original article.
books_health