 Blooming marvellous: cacti are among the few plant species that can thrive in the desert. Alan Levine/Flickr, CC BY-SA
Blooming marvellous: cacti are among the few plant species that can thrive in the desert. Alan Levine/Flickr, CC BY-SA
Sunlight, harnessed by plants in the process of photosynthesis, powers almost all life on earth. Special adaptations allow certain plants to store up a battery of carbon dioxide overnight for use in photosynthesis during the day, giving them a juicy advantage in dry desert conditions.
The processes that constitute life – such as growth, repair, movement and reproduction – all require an energy source. The immediate source of this energy for many living things is chemical energy.
High-energy carbon-based molecules, such as sugars and fats, are broken down to power the processes of life. These high-energy molecules don’t naturally occur in the environment. Work-shy and dishonest organisms, such as humans, rely on stealing high energy molecules from other organisms by eating them. Ultimately, however, more high energy molecules are required to replace those broken down.
While sugars and fats sadly don’t rain down from space, energy-rich photons (the next best thing) do, in the form of sunlight. More responsible organisms than us, such as plants and algae, perform photosynthesis. This process uses energy from sunlight to regenerate high energy molecules from their breakdown waste product, carbon dioxide (CO2), which is constantly released into the atmosphere by all living things.
Get The Latest By Email
In the most common form of photosynthesis, CO2 is taken up into leaves during the day via tiny pores in the plant surface. It is then attached, or “fixed”, straight onto a sugar molecule using energy from sunlight, to be used as a source of chemical energy – either by the plant, or by the animal that eats it.
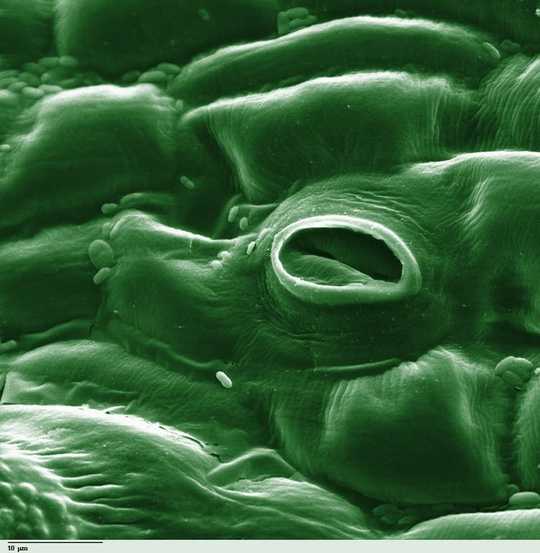 Tiny pores let carbon dioxide into the leaf – but also allow oxygen in and water out. Photohound
Tiny pores let carbon dioxide into the leaf – but also allow oxygen in and water out. Photohound
But acquiring CO2 from the atmosphere can be problematic in some situations. Opening the pores on the plant surface lets CO2 in, but also lets oxygen in and water out. Water loss is a problem in dry environments – particularly during the day, which is when CO2 is required for photosynthesis.
Additionally, in hot environments, the plant is less able to discriminate between oxygen and CO2 and can actually end up attaching oxygen to the sugar molecule. Once an oxygen molecule is fixed to a sugar, it must be prised off again at significant energetic cost, reducing the net energy that plants can acquire from photosynthesis.
Carbon dioxide batteries for efficiency
Several groups of plants have evolved that do not directly fix atmospheric CO2 to make sugars, but attach CO2 onto other molecules which can be stored, transported and broken down to release CO2 again, like a battery. This avoids the problems of water loss and accidental oxygen fixation.
Two alternative strategies have evolved to make use of this ability: C4 photosynthesis, which manipulates the concentration of CO2 in space, and CAM photosynthesis, which manipulates the concentration in time.
C4 photosynthesis is performed by 7,600 species, most of them grasses, including maize and sorghum. It has evolved independently at least 60 times, yet is present in less than 0.5% of plant species. Although highly competitive in hot environments, the energetic costs associated with carbon storage mean that plants carrying out conventional photosynthesis have the edge at lower temperatures.
C4 photosynthesis uses a special enzyme to fix atmospheric CO2 onto an acid. This enzyme is much better at discriminating between CO2 and oxygen than the classic enzyme used in traditional photosynthesis. The acid is transported deep inside the plant, where concentrations of oxygen are much lower, and the CO2 is re-released. In this low-oxygen environment, the plant makes fewer oxygen-fixing mistakes, increasing the efficiency of photosynthesis. There is an energetic cost to this roundabout way of doing photosynthesis, but this is more than offset by the decrease in costly oxygen fixation in hot environments.
 Cacti and pineapple plants use CAM photosynthesis to stay juicy. hiyori13/Flickr, CC BY-SA
Cacti and pineapple plants use CAM photosynthesis to stay juicy. hiyori13/Flickr, CC BY-SA
The other alternative kind of photosynthesis is CAM, or Crassulacean Acid Metabolism, which predates C4 photosynthesis by at least 150 million years. This was first discovered in the Crassula family of plants but has evolved independently in many lineages of plants, totalling over 9,000 species.
Like C4 plants, CAM also stores CO2 in an acid, but it performs this reaction at night, and rather than transporting the acid molecules to a different part of the plant, it simply stores them in the vacuole – the storage area at the heart of each plant cell. During the day, when the light required for photosynthesis is available, the plant doesn’t need to open its pores: it has a packed lunch already stored in its cells. This allows the plant to perform photosynthesis without opening its pores during the day, massively reducing the amount of water lost.
This is how CAM plants such as cacti and pineapples can remain succulent and watery despite the hot environments they grow in. In wetter or cooler environments, however, the problems solved by CAM and C4 photosynthesis aren’t as severe – and the energetic cost of storing and re-releasing CO2 means the plants are only competitive with their traditionally photosynthesising cousins in hot or dry environments.
Perhaps the very last place, therefore, that one might expect to find CAM plants is underwater, a pretty wet environment by all accounts. It was with some surprise therefore that CAM was first reported in the lake plant Isoetes followed by discoveries in four other genera of aquatic plants.
 Tiny aquatic plants of the genus Isoetes carry out CAM to concentrate carbon dioxide in the underwater world. US Fish & Wildlife Service
Tiny aquatic plants of the genus Isoetes carry out CAM to concentrate carbon dioxide in the underwater world. US Fish & Wildlife Service
Despite their very different environments, plants in lakes and deserts ultimately share the same problem –- the difficulty of acquiring CO2. While a lot of CO2 can be dissolved in water, it diffuses far more slowly than in air, so the water around a plant can become depleted of CO2. Aquatic plants have evolved CAM photosynthesis so that they can continue taking up CO2 at night, using it to supplement that which they can acquire during the day.
In addition to research aiming to introduce C4 photosynthesis into rice, there has been significant interest in modifying crop plants to perform CAM photosynthesis so they can better survive droughts caused by climate change.![]()
About The Author
Daniel Wood, PhD student in Plant Biology, University of Sheffield
This article is republished from The Conversation under a Creative Commons license. Read the original article.
books_gardening







